PREFER PAVING: GENE THERAPY IN HAEMOPHILIA
An online learning to inform patients and assess patient preferences.
PREFER is a 5-year, IMI-funded, project that aims to investigate how and when patient preferences regarding the benefit/risk of drugs can be used in the medical product lifecycle. As part of the project, researchers are evaluating various research questions in clinical patient preference case studies.
- The study objective
- The concept, benefits and risks of gene therapy
Rare disease
Haemophilia is a rare genetic bleeding disorder occurring in 1 of 10 000 births. There are two types, A and B, caused by different gene defects. These genetic errors cause patients to bleed longer. These bleeds can cause a variable range of other medical issues.
Lower burden of treatment
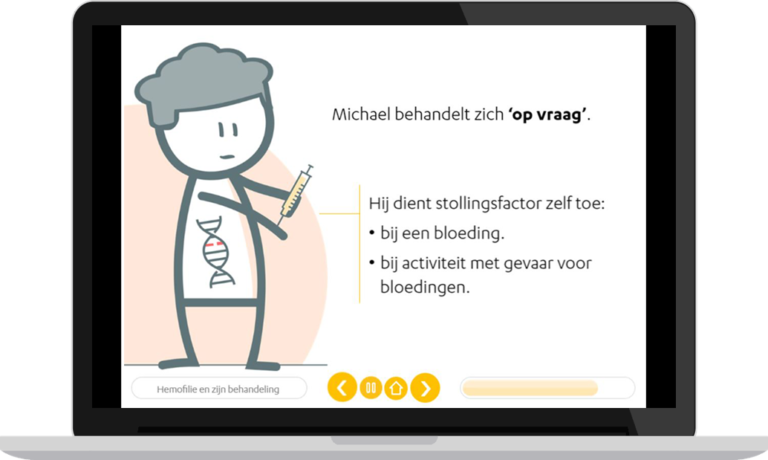
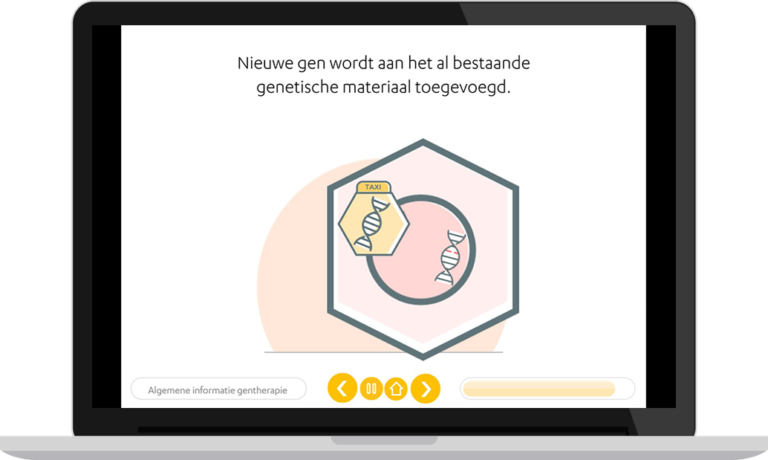
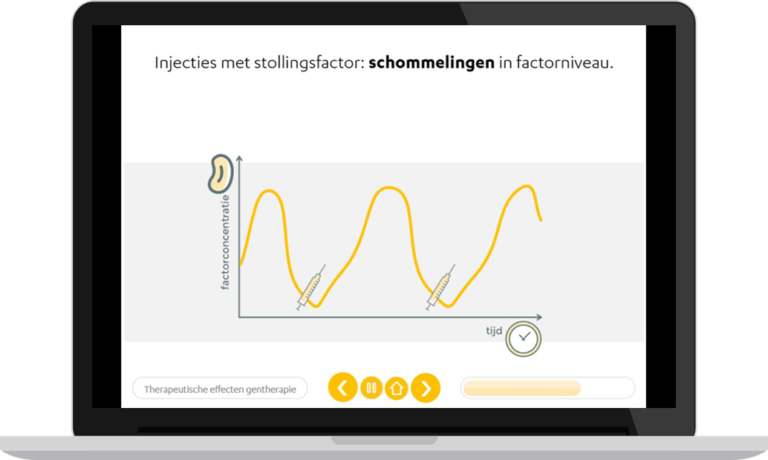
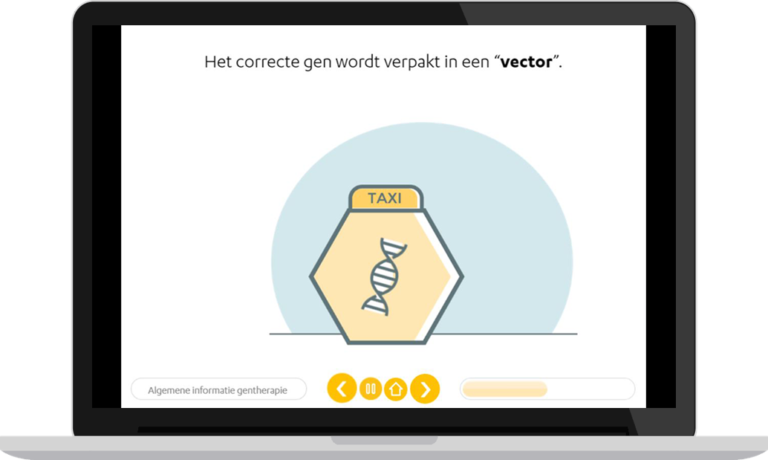
Current therapies are based on increasing coagulation factor concentrations through factor replacement. Invasiveness of this therapy (intravenous injection) and high administration frequency causes a high burden of treatment. Recently, gene therapy for the treatment of haemophilia has been developed. This seems very promising and may be able to cure patients, without such a high burden of treatment. However, it is currently still unknown what long-term side effects may occur and whether the therapeutic effect of gene therapy will be maintained throughout the full lifespan of patients.
Choice between SoC & gene therapy
In this Patient preferences to Assess Value IN Gene therapies (PAVING) case study, the team evaluates how features of the standard therapy (SoC) and gene therapy influence patients’ choices between both.

