BREAST CANCER PROMs TOOL (Co'moon)
Innovative digital tool to improve the quality of life of women with breast cancer, who are struggling with the burden of their adjuvant endocrine therapy (AET).
In short
By supporting self-management of complaints related to adjuvant endocrine therapy, our innovative digital tool empowers breast cancer survivors. The tool invites them to take matters into their own hands and helps them to better cope with their complaints and discuss them with a health care professional, partner or friend. This can improve their quality of life, which was previously negatively impacted because of the burden of treatment. Enhancing quality of life and treatment satisfaction may increase adherence and in turn, extend survival.
By building a digital tool that is evidence-based and truly engages users to participate and explore in a safe virtual environment, habit building is triggered and healthy coping behaviors can be taught. While a physical brochure can easily be put aside and forgotten, this innovative digital tool captures users’ attention and enhances message memorization and formation of healthy coping behaviors. Also, offering users the opportunity to complete patient-reported outcome measures provides important input on their experience over time, which is accessible by healthcare professionals directly in the electronic medical record.
Need for side effect management
As more and more effective therapies are available for women with breast cancer, more and more people can call themselves breast cancer survivors. In hormone-receptor positive disease, adherence to adjuvant endocrine therapy (AET) is associated with increased survival. Unfortunately, this therapy should be taken over a long period of time and can cause side effects that have a major negative impact on daily life and therefore also on treatment adherence and quality of life. As such, there is a need to support women to cope with these side effects.
Improve self-management
The overall goal is to improve the quality of life of women with breast cancer taking adjuvant endocrine therapy. The focus lies on self-management and communication. This includes learning to cope, having a different perspective on symptoms, motivating behavior change, and making sensitive subjects discussible.
How to handle side effects?
To achieve this goal, we developed a digital tool named Co’moon (“C’mon” + “hormoon”), together with UZ Leuven and breast cancer survivors, funded by Fonds voor Wetenschappelijk Onderzoek (FWO)/Kom op Tegen Kanker (KOTK).
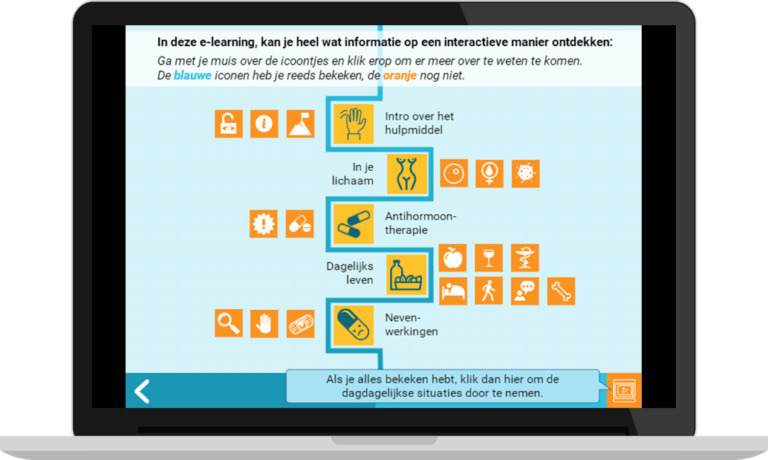
Co’moon contains 3 parts:
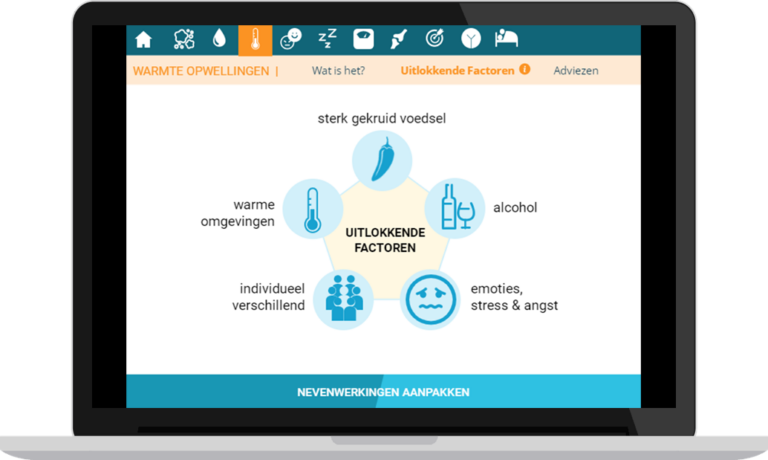
- E-learning, elaborating on adjuvant endocrine therapy (AET): what is it and what are the possible side effects. The content is based on the AET brochure available at UZ Leuven.
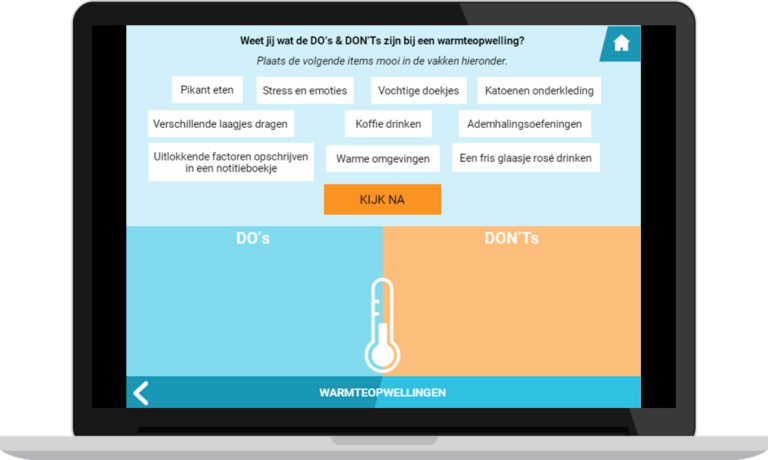
- Scenario-based serious game, offering daily life scenarios related to potential impacts of AET. Based on how users handle the scenarios, they receive feedback based on motivational guidelines about how this could affect them, their disease, and their environment.
- Patient-reported outcome measures (PROMs) questionnaire, evaluating the user’s experience over time. This is included in the patient’s electronic medical record.



Integration into electronic patient record
Co’Moon is hosted on our own MindBytes Online Platform (MOP) and directly accessible from the electronic patient record tool MyNexuzHealth. This makes it easy for women with breast cancer to access the tool, without the need for a separate login. This feature was highly appreciated among the participants of the randomized controlled trial (RCT).
Prototype, Pilot and RCT
A prototype for Co’moon was developed during a master thesis in 2017 at KU Leuven and was positively received by people with breast cancer during a pilot study. The digital tool was adapted based on the pilot study feedback. During development, the tool has been approved by nurses, oncologists, and clinical advisors. The improved tool has been tested in an RCT, comparing the use of Co’moon with the standard of care (SoC) at UZ Leuven (2021-2022).1 Several outcome measures were used, including PROMs.
1 Database of clinical trials (National Library of Medicine): Co’Moon for Supporting Breast Cancer Patients on Adjuvant Endocrine Therapy https://clinicaltrials.gov/ct2/show/NCT05085678
RCT Outcomes
Around 80 women with breast cancer and 20 healthcare professionals participated in the RCT at UZ Leuven. While the RCT did not show a comparative quality of life benefit versus controls, both breast cancer patients and clinicians expressed positive perceptions regarding the usefulness and value of Co’Moon. Notably, greater usage of Co’Moon was linked to increased benefits. Additionally, Co’Moon facilitated holistic discussions between patients and clinicians through patient-reported outcomes (PROs). This underscores the potential of Co’Moon and similar tools to be highly beneficial for a subgroup of individuals seeking or requiring supportive resources.2
2 Vanhoudt R, Coolbrandt A, Wildiers H, et al. A randomized controlled trial evaluating the role of a “patient reported outcome”-based interactive supportive tool for breast cancer patients using adjuvant endocrine therapy. Poster presented at: European Breast Cancer Conference (EBCC); March 2024; Milan, Italy. doi.org/10.1016/j.ejca.2024.113696

